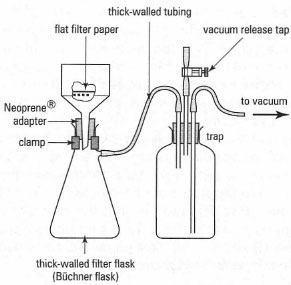
Vacuum filtration method is primarily used for a small batch of the solution to quickly dry out small crystals. The filter paper is placed over the perforated base of a Hirsch or Büchner funnel and.

Question Video Identifying The Type Of Filtration An Apparatus Can Be Used For Nagwa
Gravity filtration is used for hot solutions and impurities should be obvious.
. If you have a large volume to filter gravity filtration is probably the better choice. What is the difference between gravity and vacuum filteration. I used one myself for at-home vacuum filtration and it seemed to achieve quite low pressures.
After crystallization place beaker in waterice bath. Vacuum filtration is faster than gravity filtration because the solvent or solution and air is forced through the filter paper by the application. Gravity filtration is often used in chemical laboratories to filter precipitates from precipitation reactions as well as drying agents inadmissible side items or remaining reactants.
Typical vacuum filters operate at 8 12 in Hg vacuum or 3-5 PSI differential pressure with some of Polytechs high performance designs working at up to twice that range. Gravity Filtration also known as simple filtration for when you need to isolate the filtrate the liquid. But compare with gravity filtration it is much faster in the result of the solvent and air being forced through the filter paper by the application of reduced pressure.
Gravity filtration is used for hot solutions and impurities should be obvious. While it can also be used to separate out strong products vacuum filtration is more commonly used for this purpose. The impurity can be a drying agent or an undesired side product or leftover reactant.
The apparatus needed is. Gravity Filtration also known as simple filtration for when you need to isolate the filtrate the liquid A basic filtration under gravity is used when you are removing the solid precipitate to isolate the filtrate the liquid for further work or testing. A filtration procedure called hot gravity filtration is used to separate insoluble impurities from a hot solution.
Vacuum filtration is often used to speed up the filtration process. The filtration of hot solutions through a funnel and fluted filter paper is often carried out as part of a recrystallisation. Hot gravity filtration is commonly used to remove these impurities from a solution prior to recrystallization.
You attach a hose screw it into your faucet let the water flow and air is sucked through the hose via the Venturi Effect. The setup is shown here. Your chemists have created a slurry and have separated the product most likely using laboratory filters or maybe a laboratory centrifuge.
Begingroup Not to answer your question but perhaps to solve your problem. I have also seen vacuum filtration not filter small particles where a gravity filtration works fine. Collect crystals by vacuum filtration.
You may also use a spatula to agitate the precipitate on the filter paper for thorough drying or to remove the precipitate after the filtration is finished. Due to the fact that reduced pressure is used in this procedure special attention has to be paid to the equipment used in this procedure. The process has advantages and disadvantages in comparison to gravity filtration.
Vacuum filtration is used primarily to collect a desired solid for instance the collection of crystals in a recrystallization procedure. This allows much higher differential pressure capability across the filter media than gravity bed filters. VsVacuum filtration that is for cold solution.
How much solution is there to filter. 2 Suction filtration is more efficient at removing residual liquid leading to a purer solid. Gravity filtration can be used to collect solid product although generally vacuum filtration is used for this purpose because it is faster.
A vacuum filtration is usually faster than a simple gravity filtration using a simple conical funnel. Hot filtration is necessary for recrystallization when impurities exist in solution. This allows much higher flow rates per square foot of filter.
Vacuum filtration is fast but if you need to empty the flask part way through you. Do not heat organic solvents in a Bunsen burner use a hot plate or use a hot water bath that is on a hot plate. Gravity filtration is generally carried out to remove impurities rather than to isolate solids.
Gravity filtration will not remove all traces of the solvent and dissolved impurities. Remember never use vacuum filtration to filter a solid from a liquid if you want to use the liquid and the solvent boiling at about 125 degrees or lower will boil off in the vacuum flask while the pressure is. As the temperature changes the solute particles begin to come out of solution leaving the more soluble impurities in solution.
These units are great for demonstrating the effectiveness of the new product. You can buy a plastic Venturi Pump online for less than 20. 1 Suction filtration is much faster than gravity filtration often taking less than one minute with good seals and a good vacuum source.
In gravity filtration a suspension of a solid in a liquid is allowed to flow by gravity through a porous medium such as a filter paper. Vacuum filtration will get a lot closer. Are likely best as they are faster.
Apparently vacuum can force some near. The liquid passes through the pores in the paper and the solid is retained on the paper. You would use gravity filtration because vacuum filtration uses a vacuum as the name suggests and as a result it cools the solution.
Hot filtrations require fluted filter paper and. Rinse the crystals with small portion of cold solvent. Cold filtration technique is primarily used to rapidly cool the solution to be crystallized.
However there are several points that have to be considered when performing a vacuum filtration. Gravity filtration is also best used when the solvent has a low boiling point such as ether or hexane or when the solution is hot. Hot gravity filtration and vacuum filtration in recrystallization.
When crystals begin to grow in the filter funnel during gravity filtration it can block the funnel or stop the filtration process. Pulling a vacuum on hot solutions especially flammable ones can cause all kinds of problems. Also with vacuum filtration you can rinse the crytalline product with a very small amount of cold clean solvent to help further remove leftover supernatant.
If you are collecting the filtrate solution gravity filtration methods are often preferred. A vacuum pump either an electrical type or a water stream vacuum pump. As a result you get a lower percent yield and so you use the gravity filtration.
The impurity can be a drying agent or an undesired side product or leftover reactant. The hot solution is cooled slowly to room temperature. Vacuum filtration uses either a Buchner or a Hirsch funnel.
So its sensible to take several steps before. Recrystallization is the process of obtaining pure crystals of a compound from a solution containing impurities. A filtration procedure called hot gravity filtration is used to separate insoluble impurities from a hot solution.
Make sure to not fill the funnel above the edge of the filter paper. However they may not produce the same product quality in full-scale operation. This method results in the development of very small crystals as oppose to getting big crystals by cooling the solution slowly to a room temperature.
The two main kinds of filtration used in laboratories are gravity and vacuumsuction. Gravity filtration can be used to collect solid product although generally vacuum filtration is used for this purpose because it is faster. This is the simplest kind of filtration when the solution to be filtered is poured through a filter paper in a filter funnel.
When a solution becomes cooled the solubility of A in the solution will decrease and more will precipitate out.

Lesson Explainer Filtration And Crystallization Nagwa
Filtration Basic Laboratory Procedures Ii Fundamental Laboratory Techniques

0 Comments